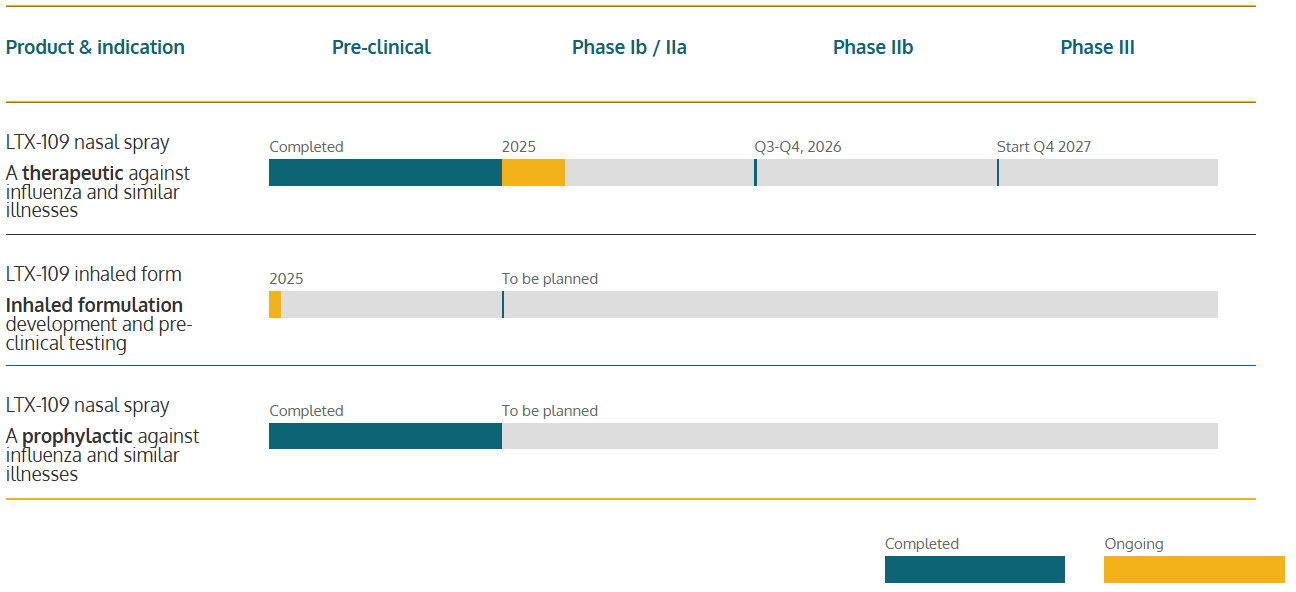
Our pipeline
This is our journey towards a future market authorization and making our drug products available for patients world-wide.
Product & indication
Pre-clinical
Phase Ib / IIa
Phase IIb
Phase III
LTX-109 nasal spray
A therapeutic against influenza and similar illnesses
- Completed
- 2025
- Q3-Q4, 2026
- Start Q4 2027
LTX-109 inhaled form
Inhaled formulation development and pre-clinical testing
- 2025
- To be planned
- To be planned
- To be planned
LTX-109 nasal spray
A prophylactic against influenza and similar illnesses
- Completed
- To be planned
- To be planned
- To be planned
- Completed
- Ongoing
Do you want to be part of our journey? See if there are available positions under Careers.