Our Science
A new approach to treating viral respiratory infections with direct-acting virucidal drugs that will transform how we choose to fight and prevent respiratory viral infections.
Therapeutic concept
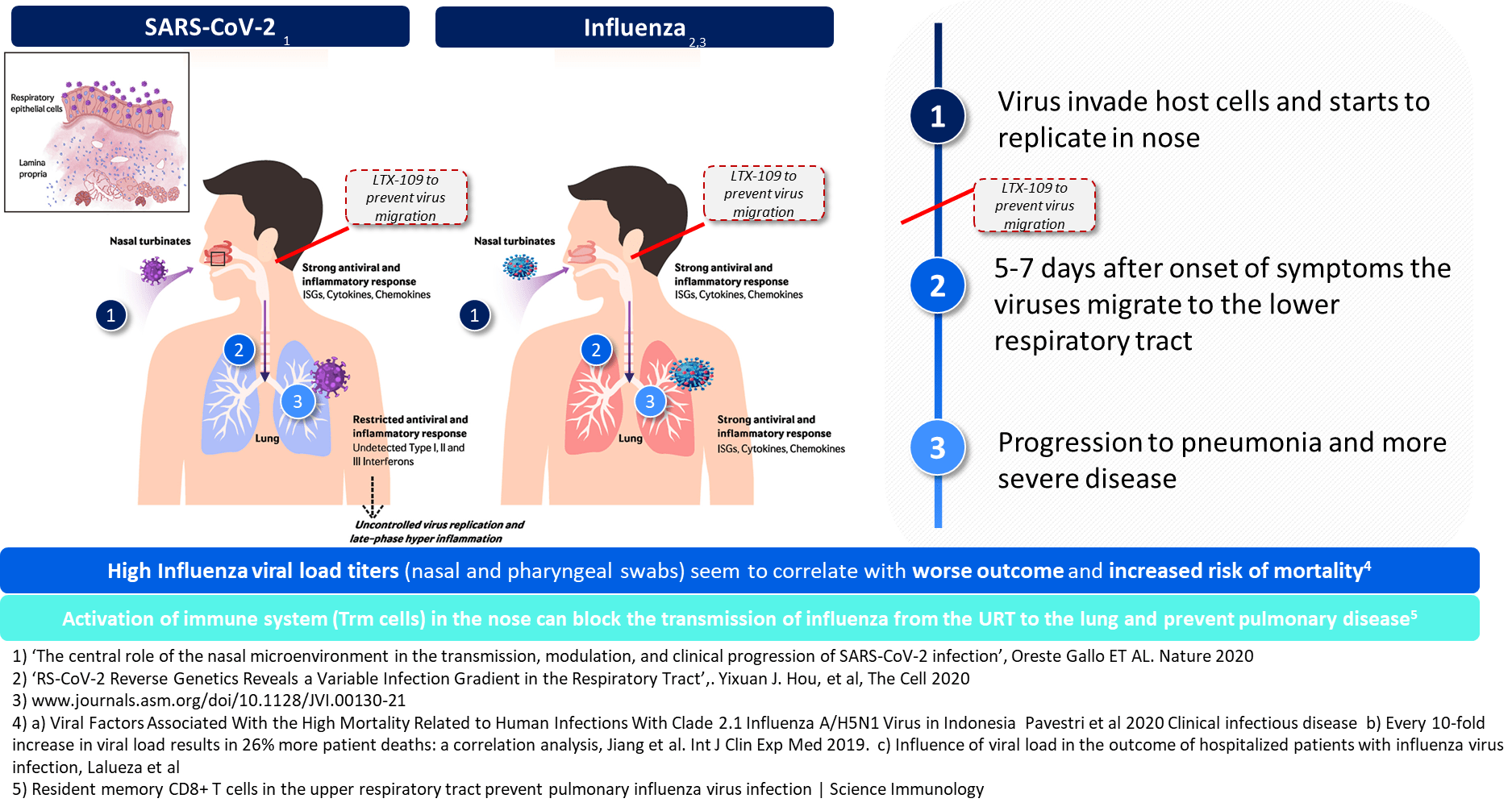
Treating infections in nose, before migration to lungs
Research has found that illnesses like SARS-CoV-2 and Influenza typically infect via the nasal route where virus replication in the nasal epithelial tissue starts. After 5 to 7 days after onset of symptoms, the viral infection migrates to the lower respiratory tract where subsequent progression of infection can lead to pneumonia and other severe complications.
Our therapeutic concept is based on treating the infection in the nasal pathway, effectively stopping or weakening the viral replication cycle prior to virus migration into the lower respiratory tract. If viruses can be killed in the upper respiratory tract, no or fewer viruses will make it alive to the later phase migration to the lower respiratory tract, hence reducing the risk and severity of a subsequent lower respiratory tract infection.
Targeted diseases and indications
Our studies have shown that many diseases are possible indications as our drug has shown efficacy against many different viruses but also against bacterias and fungi, which opens up possibilities for possible treatments.
At this stage of development, our target diseases and populations will be respiratory tract infections by influenza- and Covid-like illnesses in the high-risk patient population. This is a population where many of the available treatments show little or no significant effect, and given the lack of a fully functioning immune system vaccines will not generate the antibodies needed. We strongly believe in the strength of our direct virucidal effect that kills viruses by local treatment in the infected tissue, which can lower the sideffects compared to systemic treatments.
Disease epidemiology
Prevalence
- There are around one billion cases of seasonal Influenza annually, including 3–5 million cases of severe illness causing 290 000 to 650 000 respiratory deaths annually
- Globally, Respiratory Syncytial Virus (RSV) affects an estimated 64 million people and causes approximately 160 000 deaths each year.
- The COVID-19 (SARS-CoV-2) pandemic poses an unprecedented public health crisis, affecting billions of people. At present, however, the long-term prevalence estimates are uncertain.
Patient demographics
- People at greater risk of severe disease or complications when infected are pregnant women, children under 5 years of age, older people, individuals with chronic medical conditions and individuals with immunosuppressive conditions.
Drug efficacy
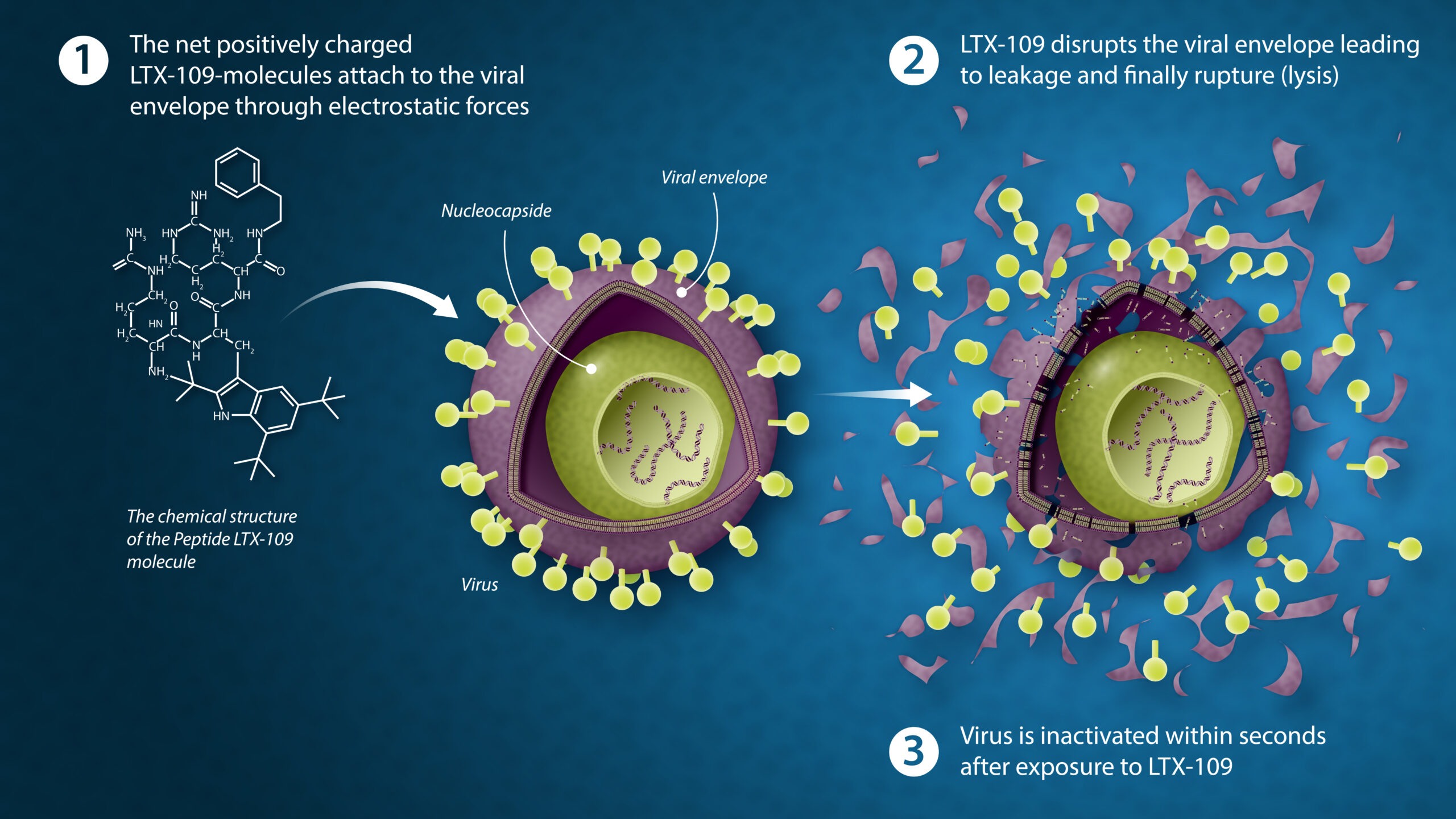
Direct virucidal effect of active ingredient
The mode of action of our drug substance LTX-109 is both rapid, selective and effective at killing viruses. These properties can be used in a strategy for treating certain viral infections such as upper respiratory infections via the directly virucidal effect. The picture below demonstrates the mechanism of action on viruses via acting on the viral envelope.
Our product breaks the membrane / envelope which causes the virus to lose its structure and function, i.e. losing its ability to further infect cells and multiply. The insides of the virus is left exposed for a possible immune response.
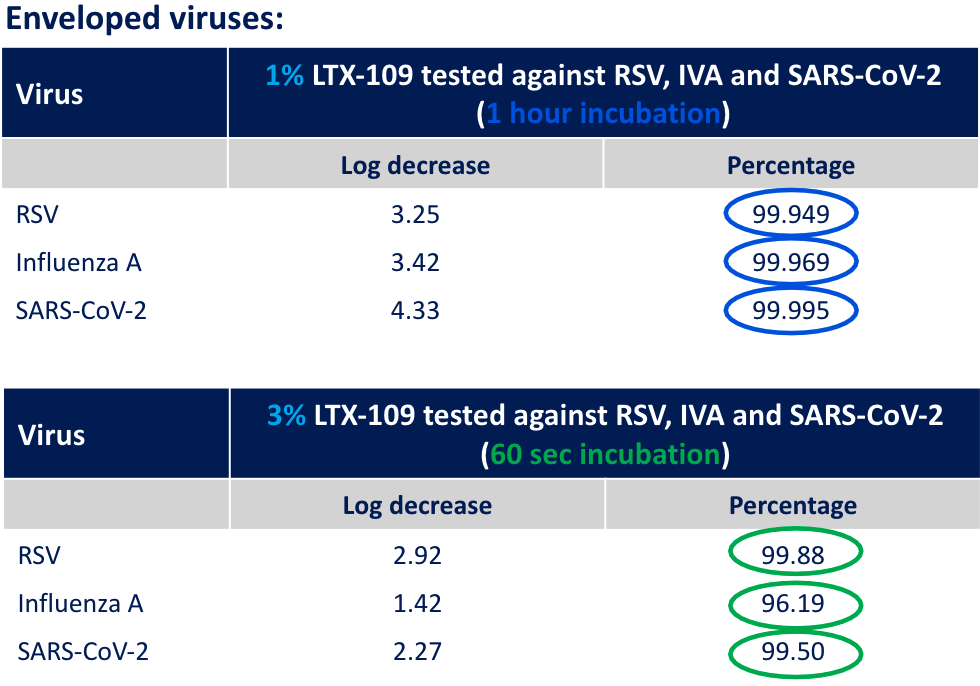
In vitro display of virucidal effect
Our drug has shown very promising results in terms of rapid virucidal effect in performed in vitro studies where three common viruses were exposed to 1% and 3% formulations for 1 hour and 60 seconds respectively. The viral reduction of > 99,9% for the 1% formulation over 1 hour and > 96% for the 3% formulation over 60 seconds shows that our drug is efficacious in killing these viruses and that the mode of action is rapid.
In vivo display of virucidal effect
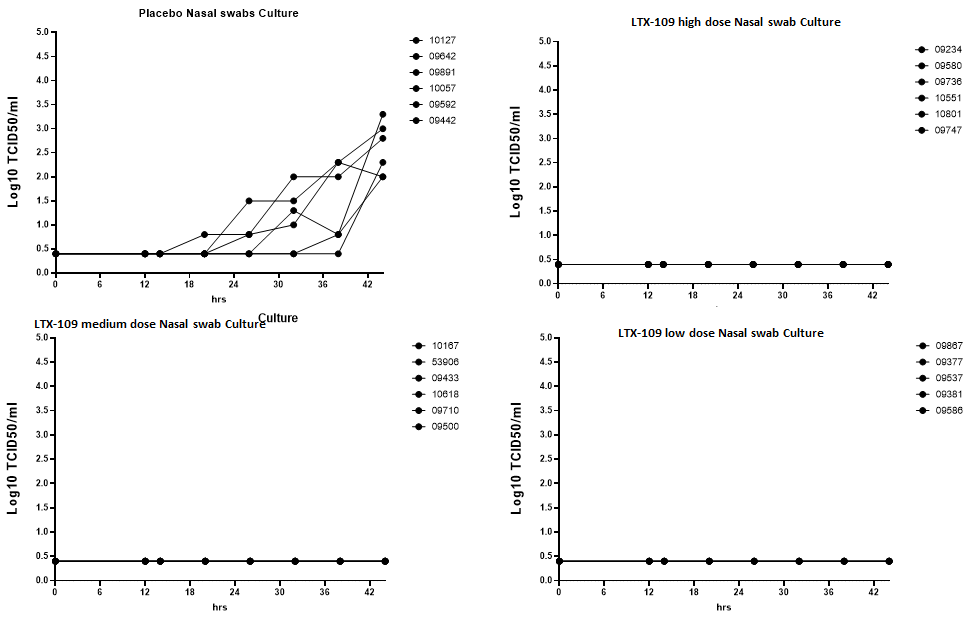
Therapeutic efficacy in a ferret model
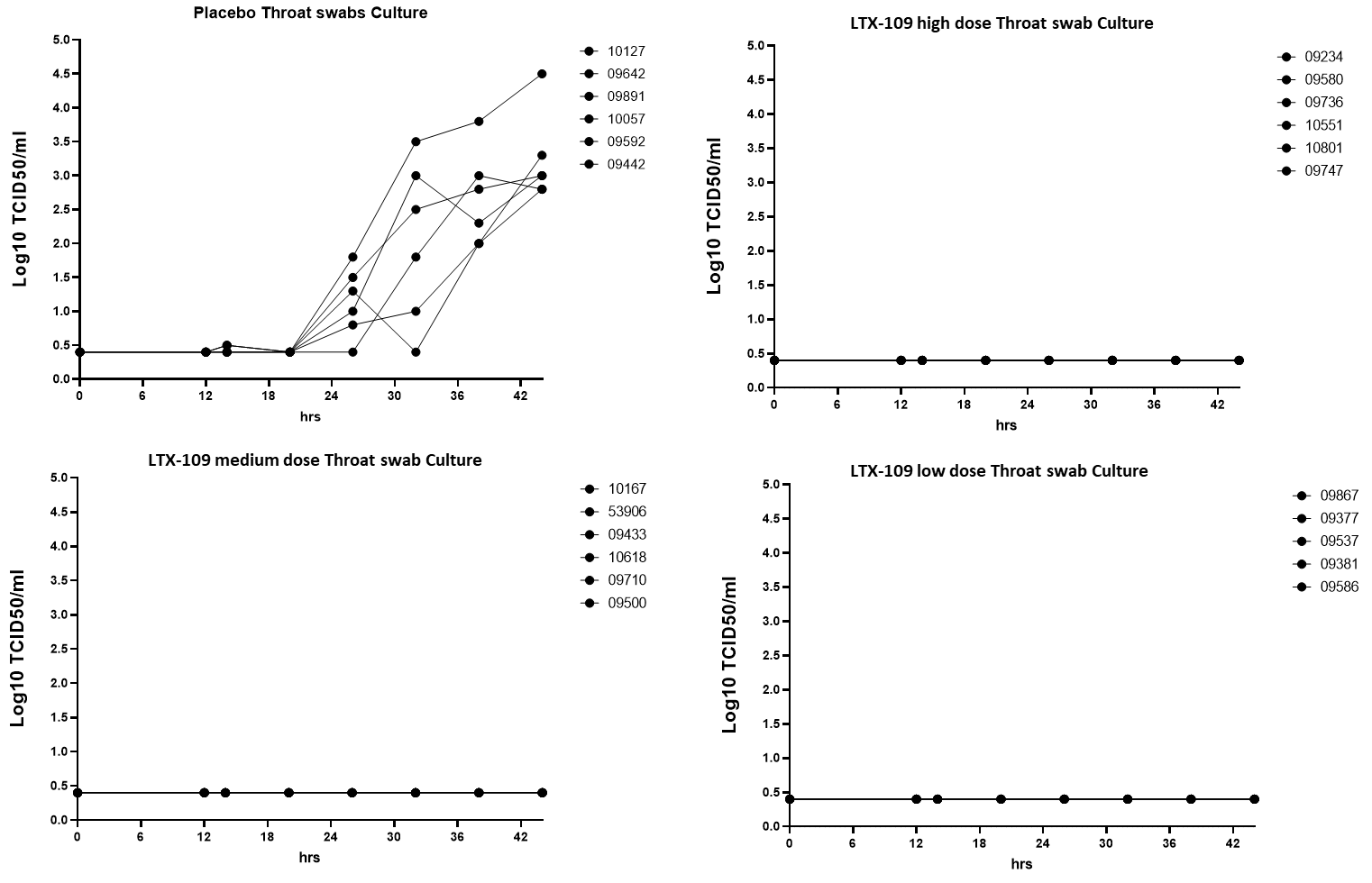
Furthering our drug into in vivo studies where ferrets were infected with SARS-CoV-2 intranasally. Subsequent treatment with our drug showed that the virucidal effect shown in vitro also could be seen in vivo where no viruses were detected in the oral swabs, the nasal cavity or in the lungs in the treated group of ferrets, but were present in measurable amounts in the control group.
Throat swabs showed that no treated ferrets had measurable amounts of viruses present, which indiates that the infection did not migrate from the nasal turbinates via the throat. The control group showed that the nasal infection, if untreated, did migrate to the throat.
Therapeutic efficacy in a hamster model
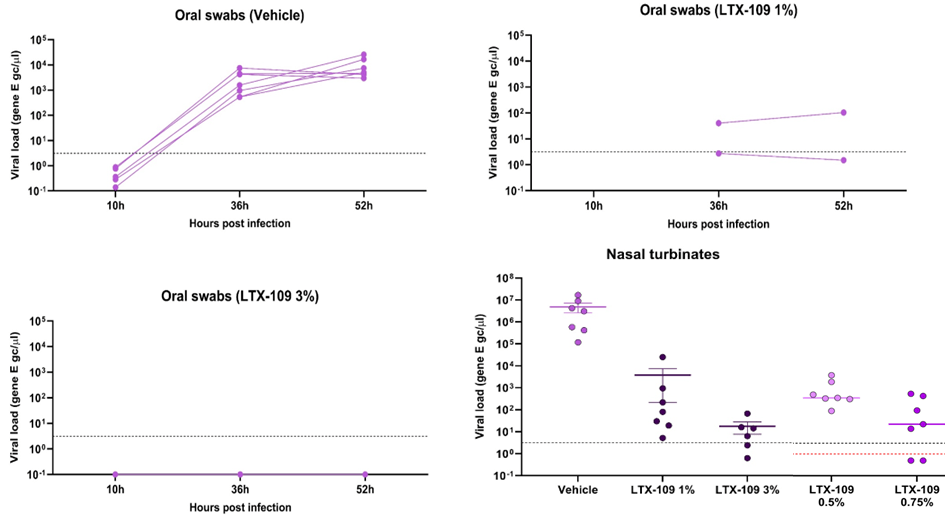
The previously observed therapeutic effect in ferrets could again be shown in a hamster model, where initially three groups of animals were treated with LTX-109 in strengths of 1%, or 3% as well as placebo for comparison. The study was then expanded to also include lower strengths of 0.5% and 0.75%. The results show a significant decrease in viral load of > 99,9%, low amounts of virus present in the oral swabs for the 1%, and no measurable viruses present in the oral swabs for the 3% group.
Prophylactic efficacy in a hamster model
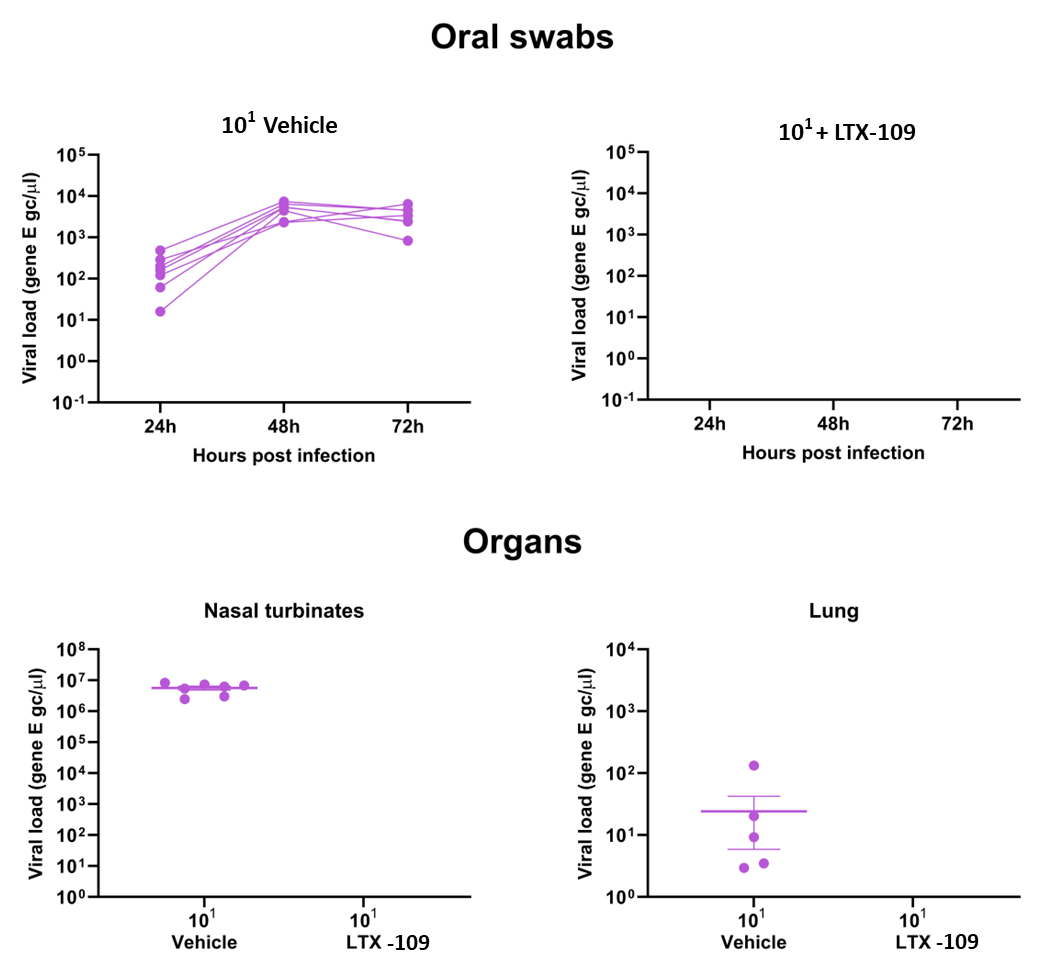
One more study on hamsters was performed to evaluate if our product has a prophylactic efficacy when administered in one dose prior to infection with SARS-CoV-2. The results showed that no treated animals had virus present in nasal swabs for up to 72 hours after infection. There were also no virus present in the nasal turbinates nor the lungs for the treated animals, whereas the control group showed high amounts of virus in the nasal swabs with a peak at 48-72 hours, as well as virus present in the nasal turbinates in high amounts, and in the lungs at lower but measurable amounts.
If you are interested in what our next steps and and what projects we have planned, click on this button to learn more about our pipeline.