Drug substance LTX-109
LTX-109 is an investigational antimicrobial drug substance with a novel membrane-lysing mechanism of action, based on the biological principle of innate immune effectors, lytic peptides. The drug has a very rapid bactericidal and virucidal activity and has shown a low propensity for resistance development. The drug has been tested in vitro and in vivo models and has undergone a comprehensive nonclinical safety and toxicology program and been studied in man in a Phase I study, six Phase I/IIa studies and one Phase II study.
Mechanism of action
The antimicrobial mode of action of LTX-109 represents a highly beneficial strategy for killing bacteria and inactivating viruses. The diagram below demonstrates the mechanism of action for LTX-109 lysis of viral envelope.
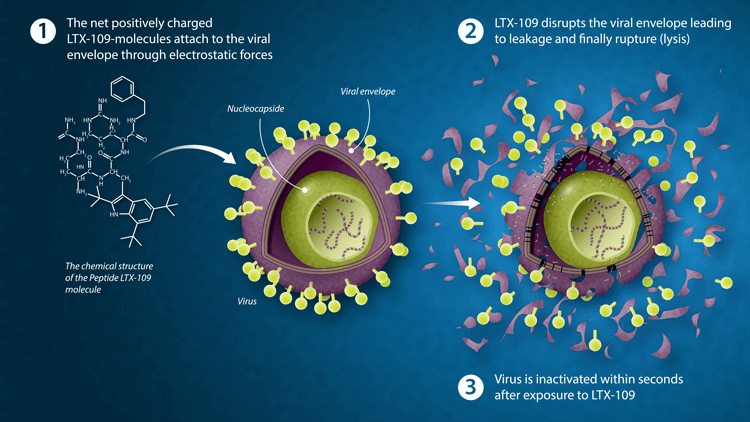
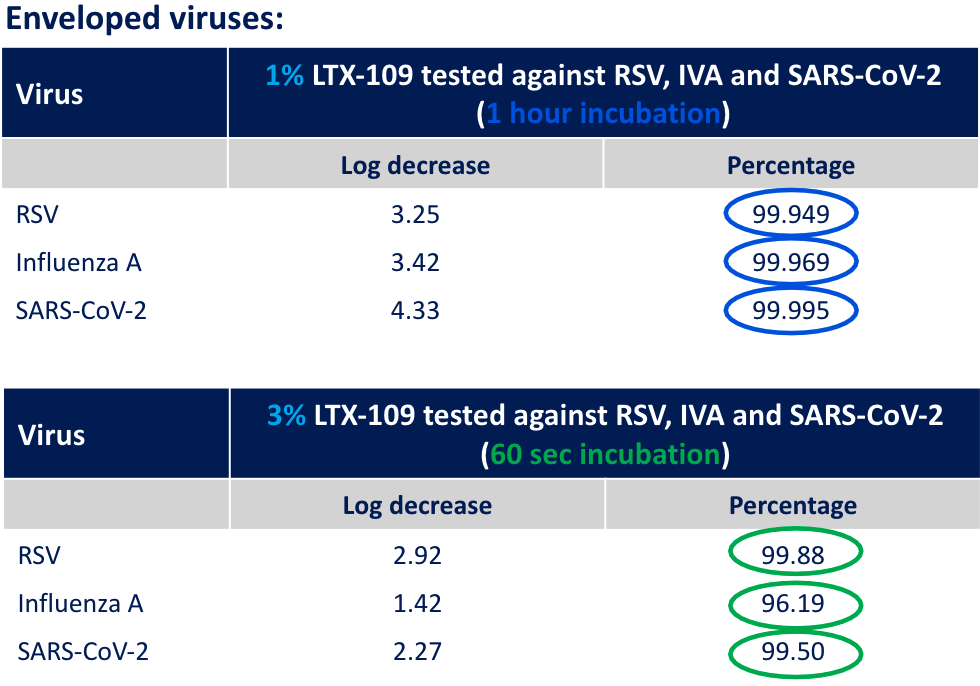
Antiviral pre-clinical highlights
Promising in vitro virucidal activity against respiratory viruses.
Established in vivo efficacy against Influenza and SARS-CoV-2

Hamster SARS-CoV-2 model – prophylactic study. Design: 28 hamsters were inoculated via the intranasal route, with 2 challenge doses of SARS-CoV-2. Swabs were collected. For each challenge dose, animals were divided into 2 groups (placebo or LTX-109 in a medium concentration formulation). The formulations were administered intranasally according to the following schedule: 20 minutes before the infection and then QID post infection.
Results: All samples were tested for the presence of the viral genome by PCR and for replication competent virus by cultivation. The study demonstrated that the prophylactic treatment with LTX-109 prevented the replication of the virus in the nose, nasal turbinates and prevented viral migration from the nasopharynx to the lower respiratory tract, irrespective of the dose of challenge dose. In the control groups viral genome was detected in all oral swabs.

Ferret influenza model- therapeutic study: Three groups of six animals and one group of 5 animals (total of 23) were intranasally inoculated with H3N2 challenge virus on Day 0. Following inoculation with virus, LTX-109 was administered a total of 9 times with treatments occurring on days 0, 1 and 2. Three concentrations (low, medium and high) of LTX-109 and one placebo group were tested. On each of day 0 and day 1, all animals were treated 4 times a day, and on day 2 all animals were treated once before euthanizing the animals for investigating both virology and pathology in respiratory tissues and fluids.
The results show that none of the animals treated with the LTX-109 had replication competent virus in their nasal turbinate swab samples while all samples from the vehicle treated group contained replication competent virus.
Antibacterial pre-clinical highlights
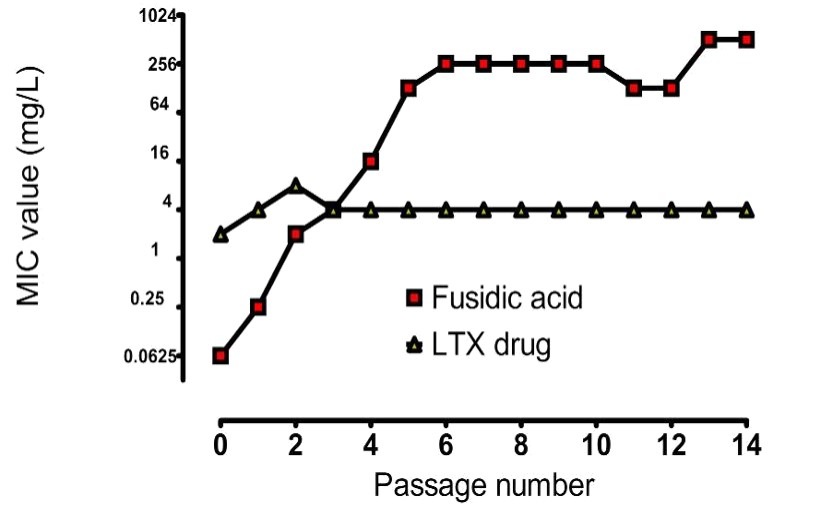
Lack of resistance development
In general, compounds acting on the cell membrane have low propensity for developing resistance. The occurrence of spontaneous resistance towards LTX-109 was studied by plating a heterogeneous mixture of bacteria. Neither in wild type, MRSA nor glycol peptide resistant Staphylococcus aureus was any spontaneous resistance observed. No signs of resistance development have been observed in 5 different Staphylococcus aureus strains after 14 passages
MIC Value
The minimum inhibitory concentration (MIC) is the lowest concentration of a chemical, usually a drug, which prevents visible growth of a bacterium or bacteria. MIC depends on the microorganism, the affected human being (in vivo only), and the antibiotic or antimicrobial peptide itself.
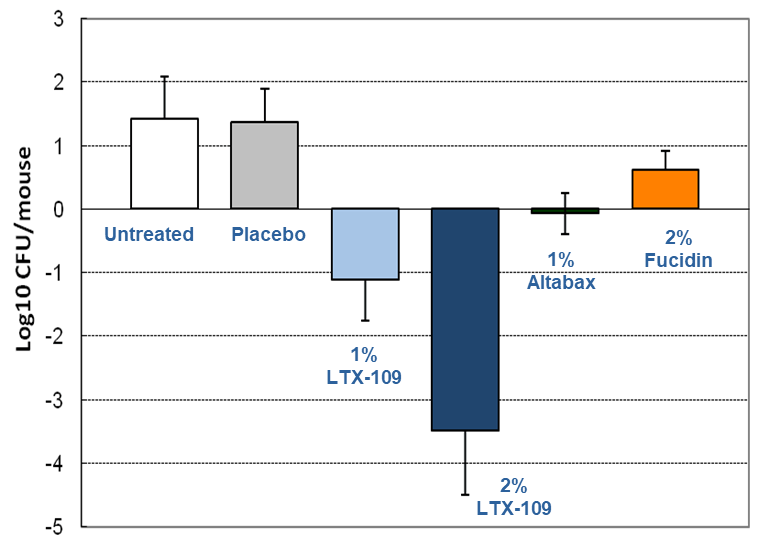
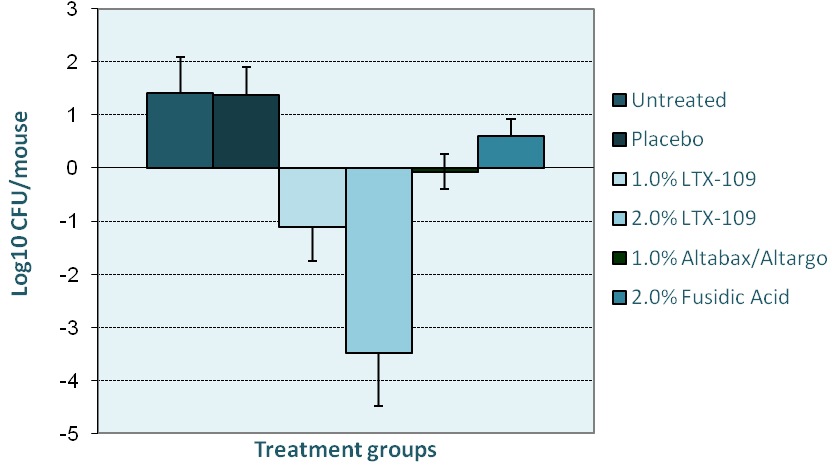
LTX-109 kills MRSA better than ‘Gold Standard’ drugs
- Murine skin infection model (tape-stripping, ATCC 33591)
- Read-out: bact growth +9 hours after teatment
- Retapamulin is a topical antibiotic developed by GlaxoSmithKline. It is the first drug in the new class of pleuromutilin antibiotics to be approved for human use. It is marketed as an ointment under the brand names Altabax and Altargo.
- Fusidic acid is an antibiotic, derived from the fungus Fusidium coccineum and was developed by Leo Pharma in Ballerup, Denmark and released for clinical use in the 1960s.
Outstanding efficiency in animal models
In vivo efficacy has been investigated at Statens Serum Institut (SSI) in Copenhagen, Denmark, and NAEJA, Canada, using a well-documented skin infection model.
A skin lesion is infected on day one, and the infection allowed to develop for 24 hours. The drug and comparators are applied 3 times in a single day. LTX-109 is strongly bactericidal against all strains of Staphylococci tested, including both hospital-acquired and community-acquired MRSA (Strain USA 300).
A biofilm can be described as a microbial colony encased in a polysaccharide matrix which can become attached to a wound surface. This can affect the healing potential of chronic wounds due to the production of destructive enzymes and toxins which can promote a chronic inflammatory state within the wound. Biofilms can be polymicrobial and can result in delayed wound healing and chronic wound infection resistant to antibiotics, leading to prolonged hospitalisation for some patients. There appears to be a correlation between biofilms and non-healing in chronic wounds. It is suggested that biofilms are a major player in the chronicity of wounds
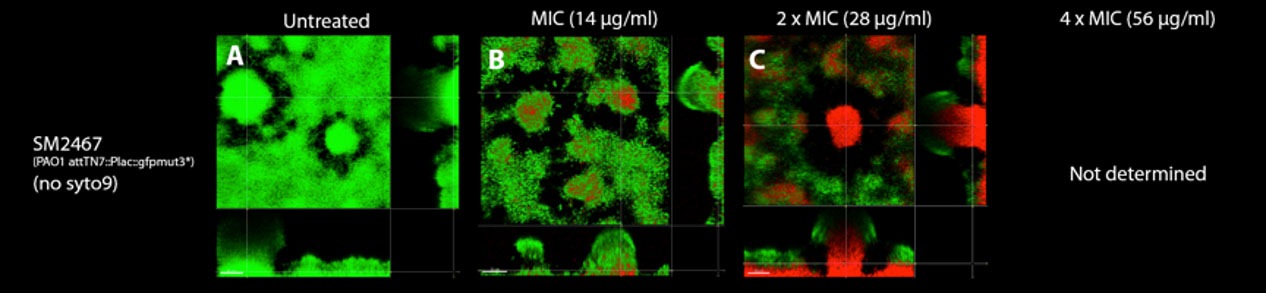
Green indicate living cells and red indicate dead cells
LTX-109 acting on Staphylococcus aureus bacteria; Live (green) and dead (red). Time lapsing inminutes